Temperature Rising Elution Fractionation (TREF) is a method for the characterization of the composition of polyolefins. It makes use of a correlation between crystallinity and composition which is present in most cases. TREF is one of the oldest methods for the characterization of polyolefins. This fact and the high scalability of the method decisively contributed to its distribution. Aside from analytical applications, preparative TREF is also common.
Temperature Rising Elution Fractionation (TREF)

Working principle
For an analytical TREF characterization, the polymer under investigation is dissolved first. As is typical for polyolefins, high temperatures (approx. 140 – 160 °C) are necessary. The hot polymer solution is transferred to a similarly heated chromatographic column which is filled with an inert support (e.g., glass beads). Inside the column, the solution is slowly cooled down. Successively, the polymer crystalizes onto the support. The more crystalline the polymer, the higher the crystallization temperature. Finally, this results in layers of polymer of different composition on the support (similar to an onion). After crystallization is finished, a constant solvent flow is applied, and the temperature of the column is again raised continuously. This allows the polymer to dissolve and materials of different composition to elute one after the other at different temperatures. By plotting the amount of eluting polymer (detector signal) against the temperature, one obtains the composition distribution of the sample.
Comparison to liquid chromatography (HPLC)
In comparison to HPLC, the high scalability of TREF is the biggest point in its favor. TREF allows to separate polymer amounts in the gram range and thus to obtain significant amounts of material for further use in relatively little time. One caveat here is that preparative TREF is performed slightly different from the description above, which makes the results not completely comparable. Thus, in the literature one may find a distinction between analytical TREF (with a support) and preparative TREF (without a support).
The advantages of HPLC lie in the significantly lower analysis time per sample and the fact that HPLC separates actually by chemical composition while TREF does so only indirectly via the crystallinity. Thus, HPLC can characterize also non-crystalline samples, while TREF cannot. Furthermore, HPLC results are not subject to crystallization-specific effects (in particular co-crystallization) which can lead to false conclusions where TREF is concerned.
Fields of application
- Characterization of the chemical composition distribution / crystallinity distribution of polyolefins
- Comparison of material compositions
- Obtaining fractions for further, particularly spectroscopic, characterization
- Investigation of the crystallization behavior of polyolefins in solution
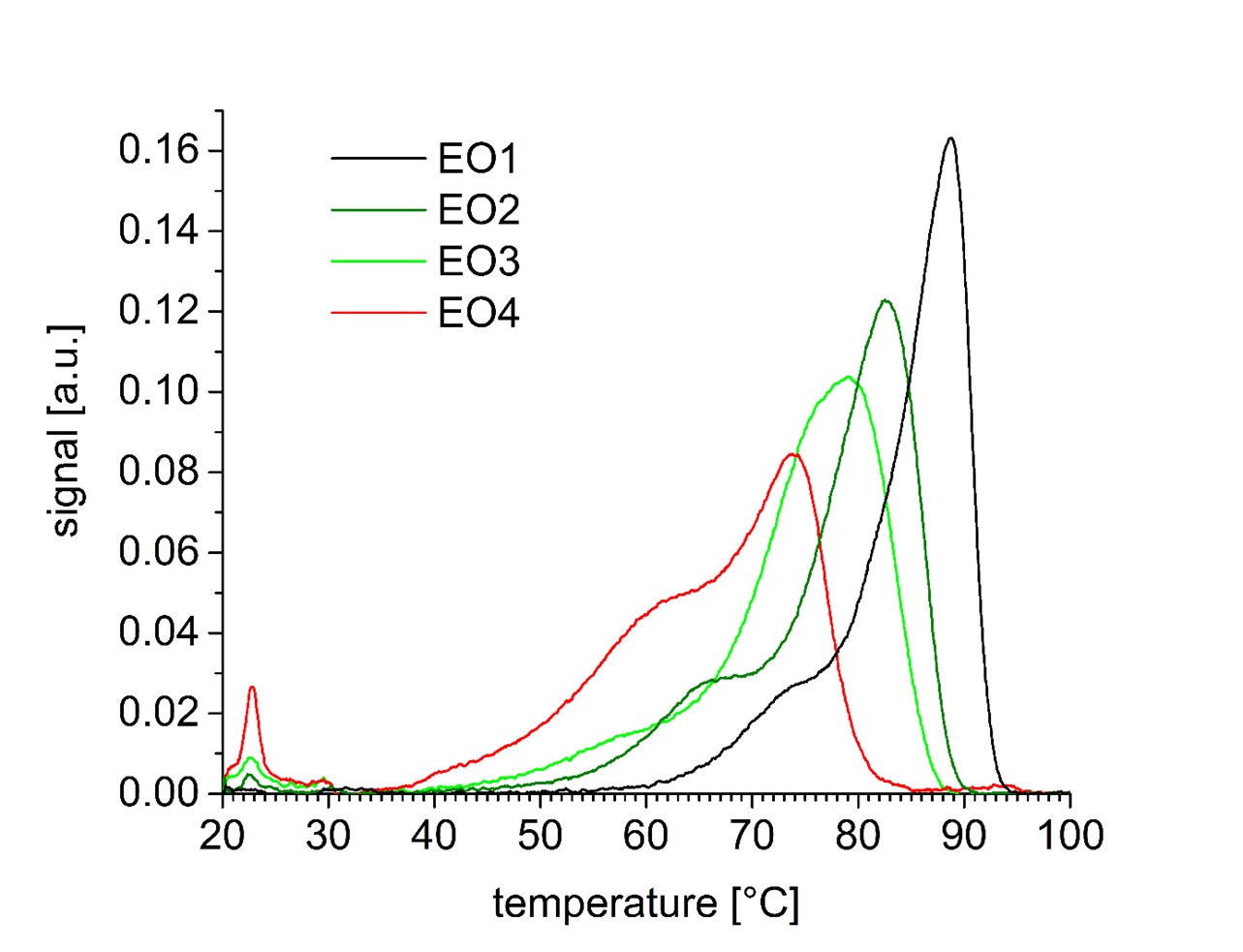
Result of TREF studies on four different ethylene-octene copolymers with different compositions:
Publications:
- J. H. Arndt, R. Brüll, T. Macko, P. Garg, J. C. J. F. Tacx, Characterization of the chemical composition distribution of polyolefin plastomers/elastomers (ethylene/1-octene copolymers) and comparison to theoretical predictions, Polymer 156 (2018) 214, DOI: 10.1016/j.polymer.2018.09.059
- V. Dolle, A. Albrecht, R. Brüll, T. Macko, Characterisation of the Chemical Composition Distribution of LLDPE Using Interactive Liquid Chromatography, Macromol. Chem. Phys. 212 (2011) 959, DOI: 10.1002/macp.201000653
- A. Albrecht, R. Brüll, T. Macko, F. Malz, H. Pasch, Comparison of High-Temperature HPLC, CRYSTAF and TREF for the Analysis of the Chemical Composition Distribution of Ethylene-Vinyl Acetate Copolymers, Macromol. Chem. Phys. 210 (2009) 1319, DOI: 10.1002/macp.200900135
- A. Albrecht, R. Brüll, T. Macko, P. Sinha, H. Pasch, Analysing the Chemical Composition Distribution of Ethylene-Acrylate Copolymers: Comparison of HT-HPLC, CRYSTAF and TREF, Macromol. Chem. Phys. 209 (2008) 1909, DOI: 10.1002/macp.200800223
 Fraunhofer Institute for Structural Durability and System Reliability LBF
Fraunhofer Institute for Structural Durability and System Reliability LBF