The term liquid chromatography refers to a variety of separation methods with a similar working principle. They all share a separation in a liquid. In the area of polymer analytics, gel permeation chromatography (GPC) and high performance liquid chromatography (HPLC) are the most important variants. Sometimes GPC is thought of as a variant of HPLC, but in the context of this text HPLC refers specifically to a method for separating according to chemical composition.
Liquid chromatography (HPLC, from room temperature up to 200 °C)

Working principle
Generally, for an HPLC analysis an analyte is pumped through a chromatographic column in a liquid (mobile phase). On the inside, the column contains a material (stationary phase) which can interact with the analyte. The strength of the interaction is determined by the identity of the analyte, the composition of the mobile phase and the identity of the stationary phase. Furthermore, temperature has an influence. Aside from this general influence, the temperature is particularly important for the analysis of polyolefins (polyethylene (PE), polypropylene (PP), …). As these materials are generally insoluble at room temperature, HPLC characterizations of polyolefins have to be performed at significantly elevated temperatures (140 – 160 °C). This is possible through the application of specific instruments as well as specific stationary and mobile phases.
Variations and applications
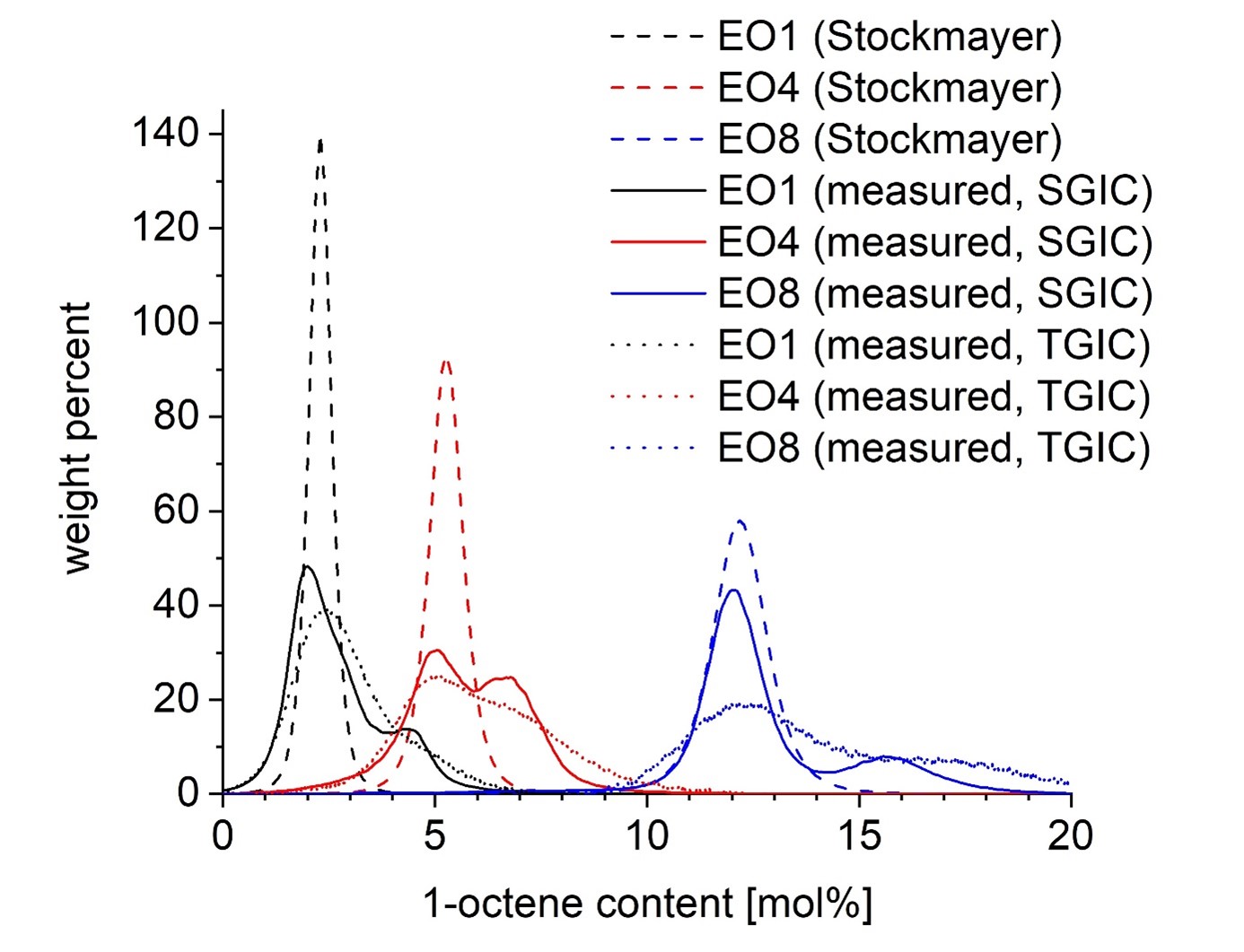
In the field of polymer analytics, HPLC separations are, in particular, employed to elucidate the composition distribution of copolymers. The method can be applied to various types of copolymers (block copolymers, grafting copolymers, random copolymers, …). Particularly in the field of polyolefin analysis, elucidating the distribution of branching (equivalent to the comonomer distribution in LLDPE) is of interest. By employing specific analysis conditions (liquid chromatography under critical conditions (LCCC), it is also possible to separate polymers according to end groups or to separate block copolymers from blends as well as to elucidate the length of the blocks in block copolymers. An adjacent field where HPLC is employed is additive analytics.
Due to their molecular size, polymers generally show a strong interaction with stationary phases. Thus, for a targeted separation, it is usually essential to vary this interaction in a controlled manner throughout the chromatographic separation. It is possible to do so by either varying the composition of the mobile phase or by varying the temperature. These approaches are referred to as solvent gradient interaction chromatography (SGIC) and temperature gradient interaction chromatography (TGIC), respectively. The application areas of both methods overlap, each having specific advantages. SGIC is more commonly applied, so far.
Fields of application
- Elucidation of the distribution of tacticities, short-chain branching content and the composition of copolymers/blends (in particular also for recyclates)
- Comparison of material compositions
- Differentiation of modified and unmodified sample components after post-reactor modification (e.g. for PP-g-MAH)
Publications:
- T. Macko, J. H. Arndt, Y. Yu, R. Brüll, Temperature gradient interaction chromatography of linear polyethylene and isotactic polypropylene, Polymer 231 (2021) 124131, DOI: 10.1016/j.polymer.2021.124131
- D. Kot, M. Zou, K. Brunnengräber, J. H. Arndt, T. Macko, B. J. M. Etzold, R. Brüll, Porous graphite as stationary phase for the chromatographic separation of polymer additives - determination of adsorption capability by Raman spectroscopy and physisorption, J. Chrom. A 1625 (2020) 461302, DOI: 10.1016/j.chroma.2020.461302
- J. H. Arndt, R. Brüll, T. Macko, P. Garg, J. C. J. F. Tacx, High performance liquid chromatography of polyolefin plastomers/elastomers (ethylene/1-octene copolymers) – Comparison of different solvent systems, J. Chrom. A 1593 (2019) pp. 73-80, DOI: 10.1016/j.chroma.2019.01.067
- T. Macko, J. H. Arndt, R. Brüll, HPLC Separation of Ethylene–Vinyl Acetate Copolymers According to Chemical Composition, Chromatographia 82 (2019) pp. 725 – 732, DOI: 10.1007/s10337-019-03697-x
- J. H. Arndt, R. Brüll, T. Macko, P. Garg, J. C. J. F. Tacx, Characterization of the chemical composition distribution of polyolefin plastomers/elastomers (ethylene/1-octene copolymers) and comparison to theoretical predictions, Polymer 156 (2018) pp. 214-221, DOI: 10.1016/j.polymer.2018.09.059
- N. Apel, E. Uliyanchenko, S. Moyses, S. Rommens, C. Wold, T. Macko, R. Brüll, Separation of Branched Poly(bisphenol A carbonate) Structures by Solvent Gradient at Near-Critical Conditions and Two-Dimensional Liquid Chromatography, Anal. Chem. 90 (2018) pp. 5422-5429, DOI: 10.1021/acs.analchem.8b00618
- N. Apel, E. Uliyanchenko, S. Moyses, S. Rommens, C. Wold, T. Macko, K. Rode, R. Brüll, Selective chromatographic separation of polycarbonate according to hydroxyl endgroups using a porous graphitic carbon column, J. Chrom. A 1488 (2017) pp. 77-84, DOI: 10.1016/j.chroma.2017.01.075
- T. Macko, J. H. Arndt, R. Brüll, Elution Behavior of Polypropylene with Different Tacticity – An Overview, Macromol. Symp. 356 (2015) pp. 77-86, DOI: 10.1002/masy.201500045
 Fraunhofer Institute for Structural Durability and System Reliability LBF
Fraunhofer Institute for Structural Durability and System Reliability LBF