Thermal Analysis

Differential Scanning Calorimetry (DSC) allows temperature and time dependent examination of thermal characteristics, such as crystallization and aging processes, the quantification of heat of reaction, determination of glass transition and phase transition temperatures as well as specific heat capacity or the analysis of enthalpic processes.
Tabbed contents
Methods and Instruments
The thermal characteristics of a sample are measured by determining the difference in heat flow between a sample and an empty reference crucible during a pre chosen temperature-time programming.
An aluminum crucible containing 5 mg to 10 mg of the sample and an identic, empty reference crucible are placed in a nitrogen flushed oven chamber and heated at a fixed rate. Thermocouples under the crucibles measure the temperature difference between sample and reference crucible, thus determining the difference in heat flow.
Changes in enthalpy of the test sample are shown, according to the thermal process, as endo- or exothermal peaks and steps.
With calorimetric measurements the temperature range of the glass transition, crystallization and melting of the sample can be determined. The glass transition can be seen by a step in the heat flow – temperature curve, crystallization by an exothermal peak, and melting of the crystalline phase by an endothermal peak.
By comparing the crystallization enthalpy with the melting enthalpy, statements regarding the degree of crystallization can be made.
DSC 823e (Mettler Toledo)
Temperature: -60 °C to 450 °C
Atmosphere: Nitrogen
Cooling rate: up to -15 K/min
Sample crucible: Aluminum (40 µl or 100 µl)
Standards
e.g. DIN EN ISO 11357, ASTM E1269
Areas of Application
Determination of:
- Glass transition temperatur
- Crystallization temperature
- Melting temperature
- Examination of crystallization processes
- Specific heat capacity
- Kinetic observation of chemical reactions
- Determination of (relative) crystallinity from the measured crystallization and melting enthalpy
- Indication of physical aging through occuring enthalpy relaxations.
- Separation of reversible and irreversible processes through temperature modulated measurements
Application Example
Examination of Polylactide
For the examination of new materials used in biodegradable plastic implants, DSC was used to examine how the processing conditions (for example injection molding parameters), the chemical structure of copolymers, the thermal storage and the in-vitro storage in a physiological solution affect the thermal properties of the material.
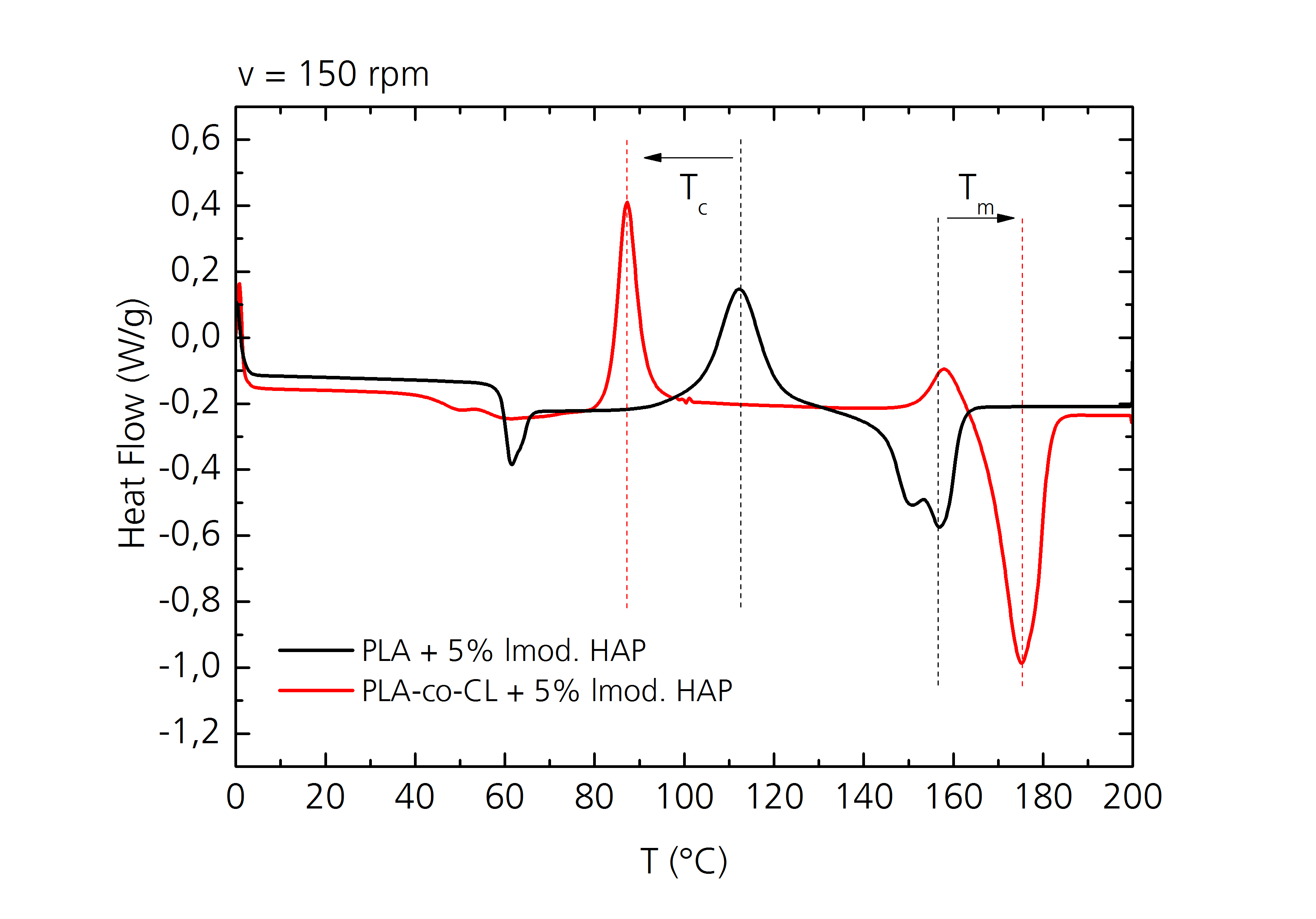
The copolymers of PLA and PCL show a decrease in crystallization temperature and an increase in melting temperature with increasing PCL content (upper figure). These are relevant factors to consider when choosing the process window for producing the samples. By choosing an appropriate temperature the crystallinity can be adjusted, which affects the degradation rate inside the body.
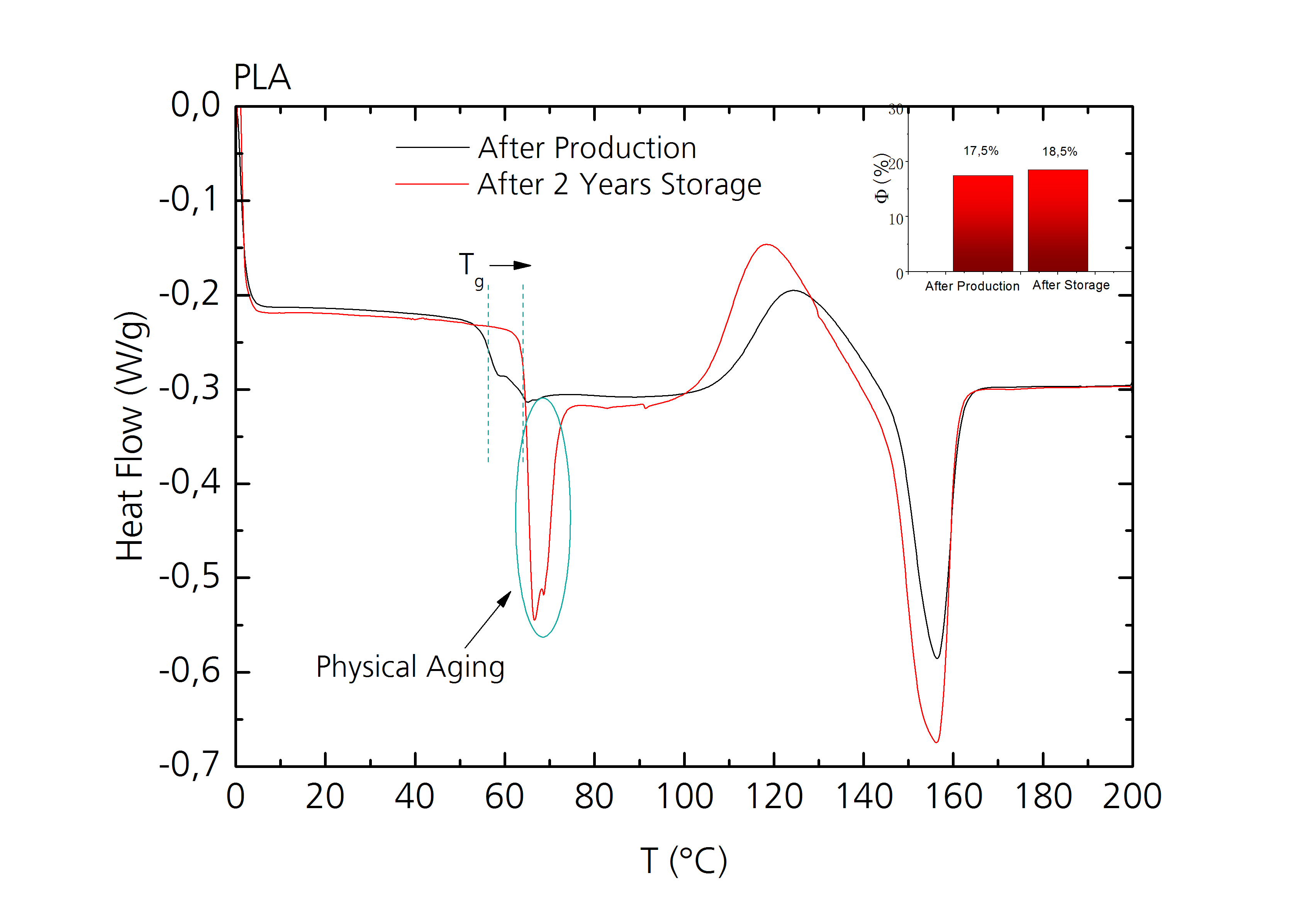
DSC measurements of a PLA-co-PCL sample directly after production and after two years of storage at room temperature show only a slight increase of crystallinity, but a significant shift of the glass transition temperature and an enthalpy relaxation peak due to physical aging of the sample (lower figure).
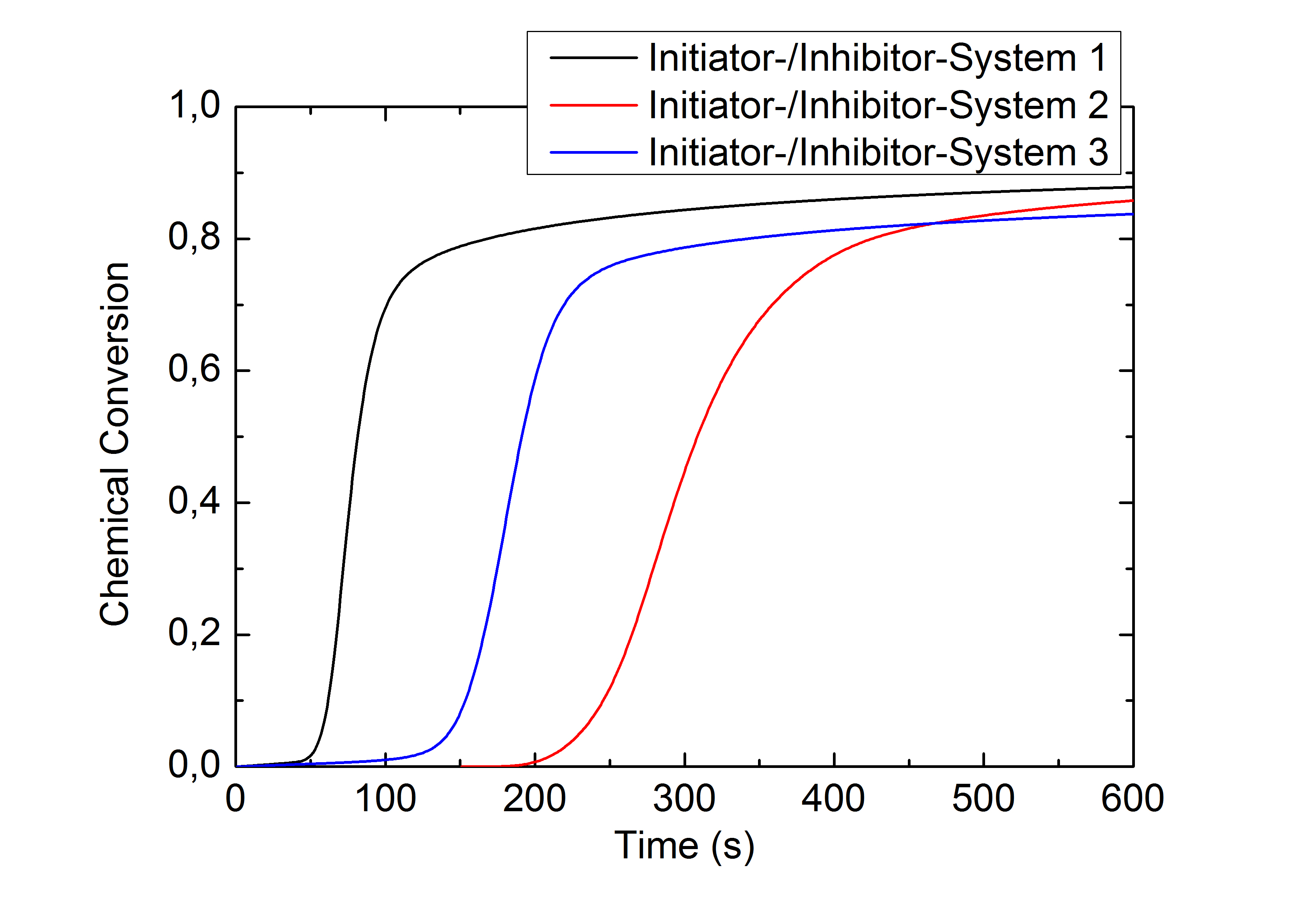
Monitoring of Chemical Reactions
Calorimetry can also be used to examine rapid chemical reactions, for example, the curing of resins. With the measured heat flow and knowledge of the sample weight inserted, the specific reaction enthalpy can and the chemical conversion rate of the reaction can be determined.
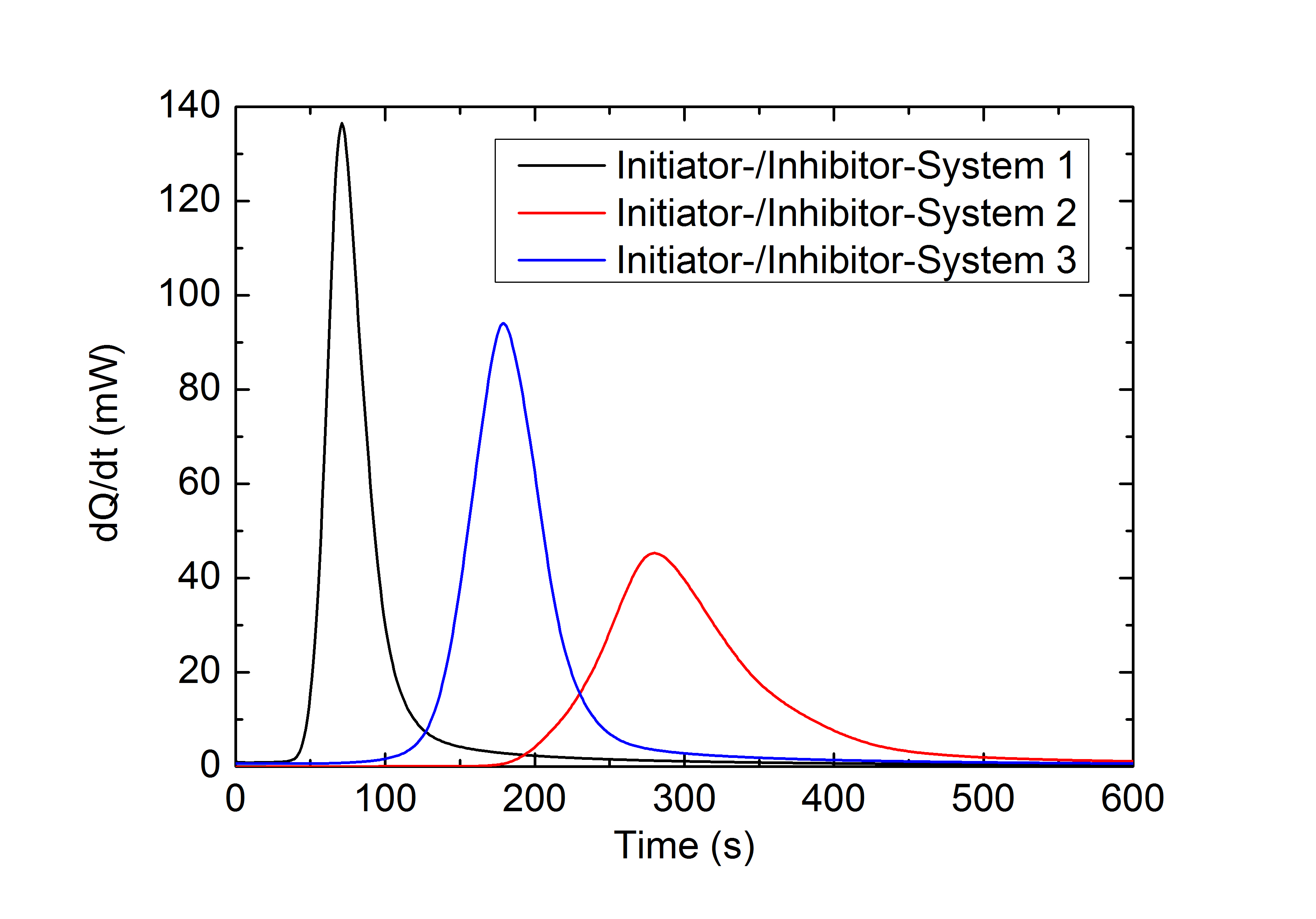
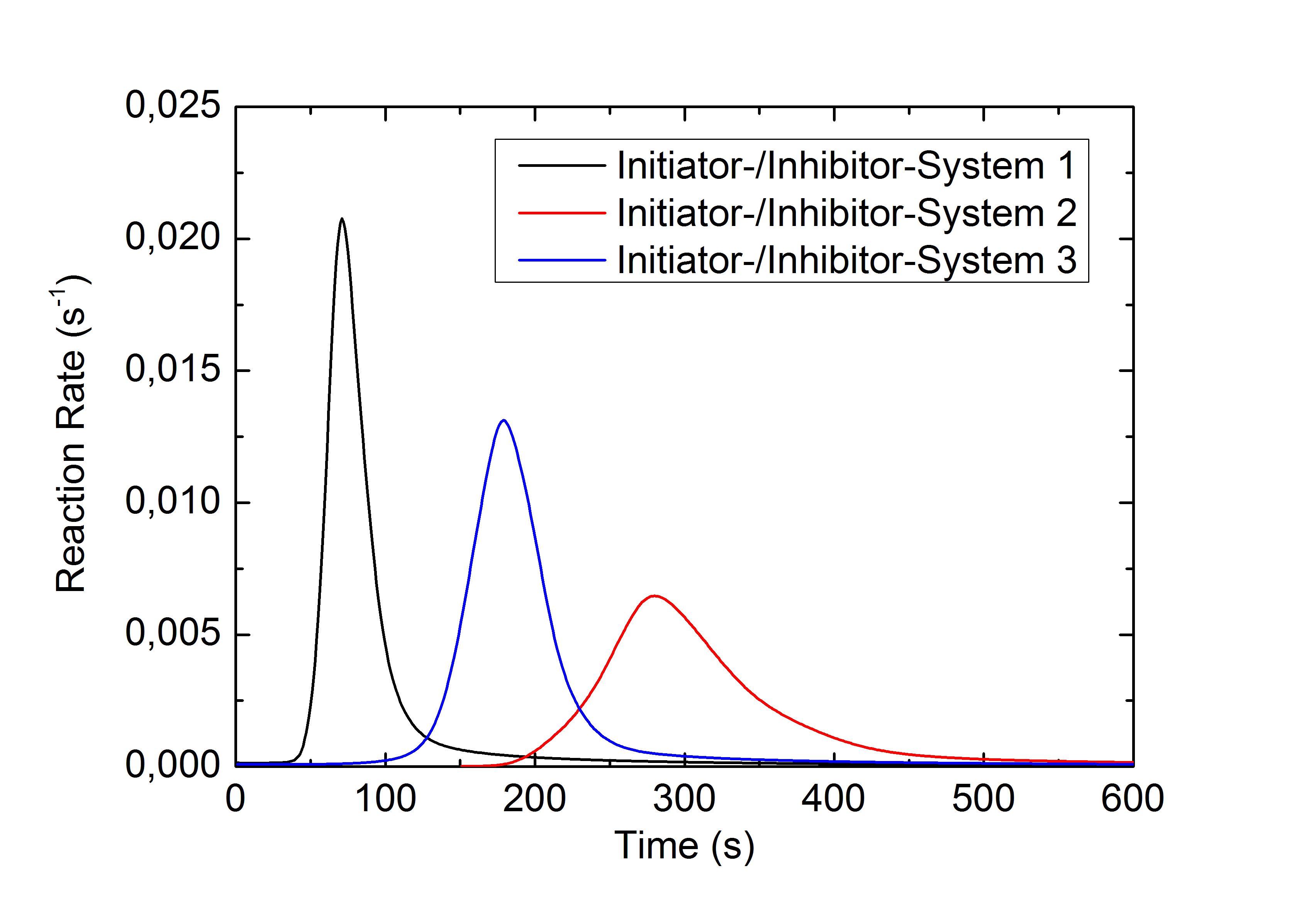
This application example illustrates the effect a variation of the initiator- to inhibitor concentration ratio in a two component methacrylate resin has on the reaction rate and chemical conversion: The upper figure shows the heat of reaction during the isothermal curing of a methacrylate resin with different initiator-inhibitor ratios. System 1 has the highest, System 3 the lowest initiator-inhibitor ratio. The reaction rates calculated from this data (middle figure) and the chronological sequence of the conversion (lower figure) show a deceleration and inhibition of the reaction with a decreasing initiator-inhibitor ratio. The final conversion of this highly crosslinked system is nearly independent from the initiator-inhibitor ratio used.
 Fraunhofer Institute for Structural Durability and System Reliability LBF
Fraunhofer Institute for Structural Durability and System Reliability LBF