Epoxy resins, polyurethanes and other reactive systems are used, for example, in electrical engineering, automotive engineering or even in the construction industry as encapsulation materials, adhesives or coatings. The good service properties of such reactive systems are countered by the disadvantages of the reaction-related volume shrinkage that occurs due to chemical cross-linking during curing. For encapsulation of components, in lithography or for medical applications (e. g. dental fillings), low-shrinkage materials are required to achieve low stresses between substrate and cladding.
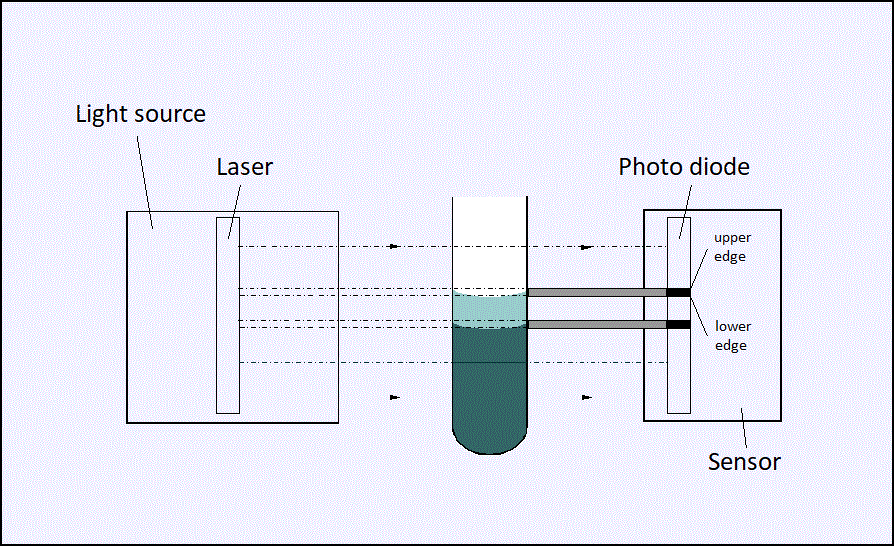
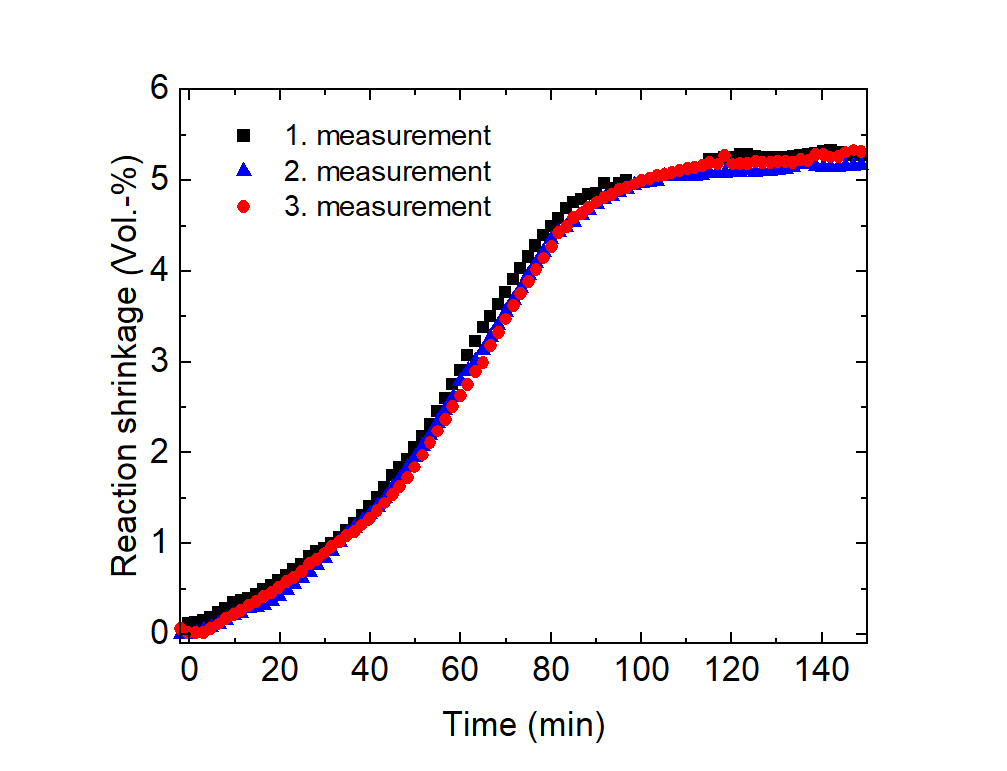
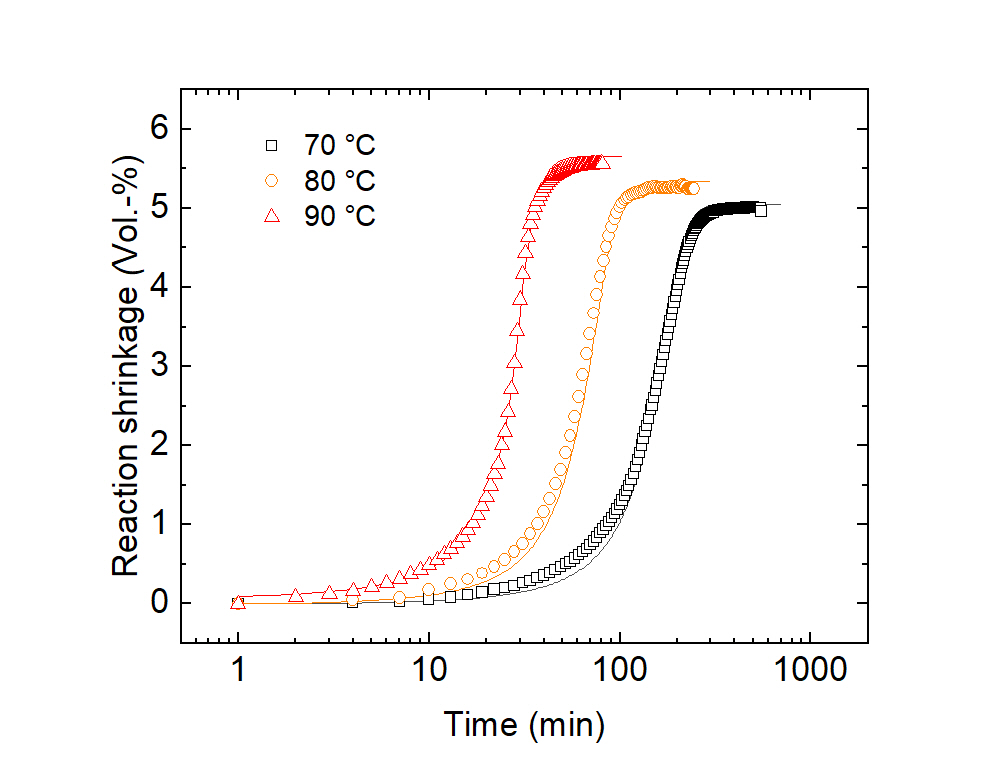
With the dilatometer developed at Fraunhofer LBF for monitoring reaction shrinkage, the volume of transparent or highly filled resin systems can be monitored isothermally during curing [1].
[1] Holst, Marco: Reaktionsschwindung von Epoxidharz-Systemen. Dissertation, Technische Universität Darmstadt (2001)